
Si definisce dipolo elettrico un sistema costituito da due cariche elettriche puntiformi q, dello stesso valore ma di segno contrario, vincolate tra loro. - ppt scaricare
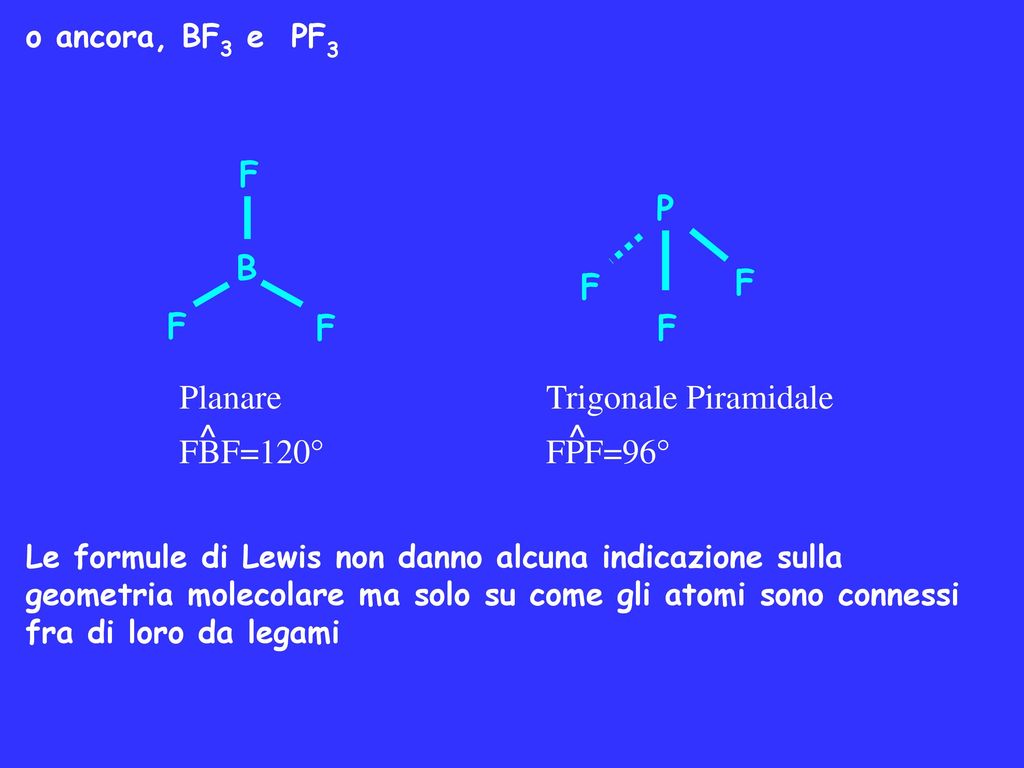
Boron trifluoride (displaystyle B{ F }_{ 3 }) is a nonpolar molecule, whereas ammonia (displaystyle N{ H }_{ 3 }) is a polar molecule. The difference in polarities is related to the

BF3 lewis structure, molecular geometry, polar or nonpolar, hybridization, Bond angle | Molecular geometry, Molecular, Vsepr theory

All three of the boron-fluorine single bonds in bf3 are polar. in which direction should the polarity - brainly.com
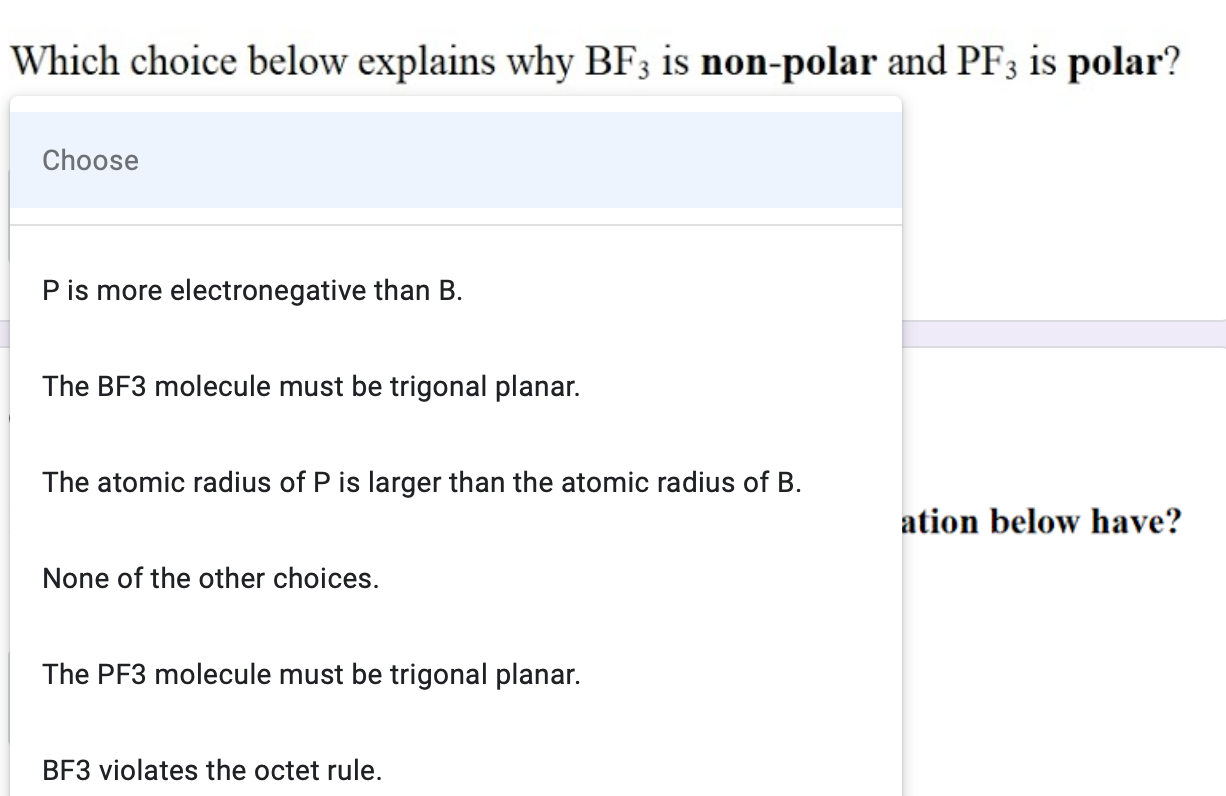
✓ Solved: The molecules BF3, CF4, CO2, PF5, and SF6 are all nonpolar, even though they contain polar...
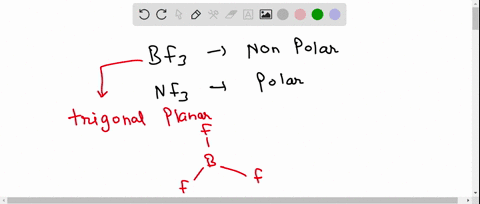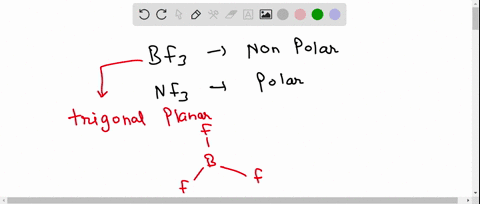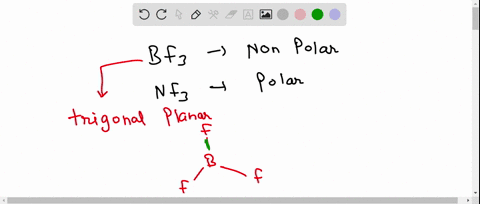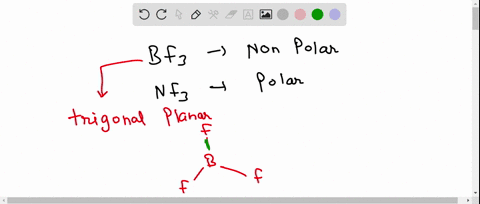
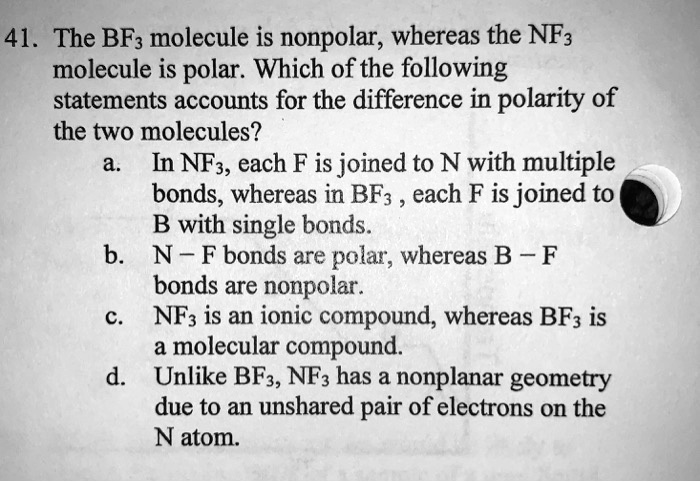
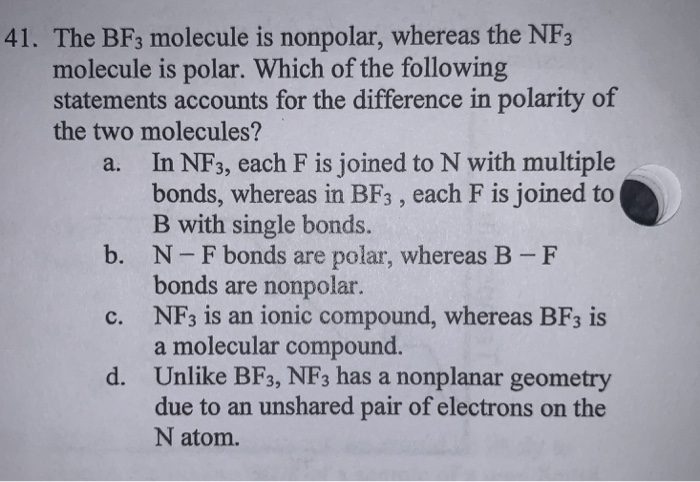
SOLVED: 41. The BF3 molecule is nonpolar, whereas the NF3 molecule is polar. Which of the following statements accounts for the difference in polarity of the two molecules? In NF3, each F
Both BF3 and NF3 are covalent compounds but NF3 is a polar compound while BF3 is non-polar. How can you explain it? - Quora
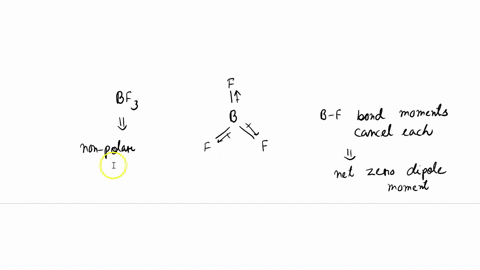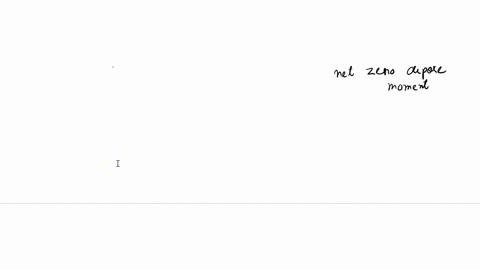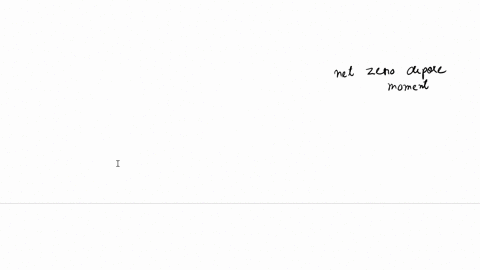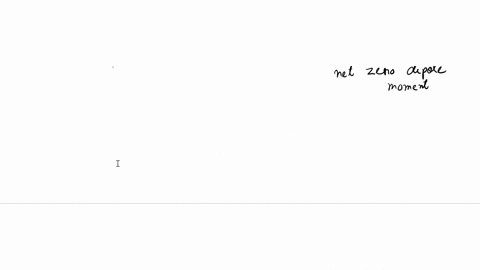
SOLVED: (a) Is the molecule BF3 polar or nonpolar? (b) If you react BF3 to make the ion BF3^2-, is this ion planar? (c) Does the molecule BF2Cl have a dipole moment?
![Best Overview on: BF3 Polar or Nonpolar [#1] - Science Education and Tutorials Best Overview on: BF3 Polar or Nonpolar [#1] - Science Education and Tutorials](http://sciedutut.com/wp-content/uploads/2021/05/bf3-3.png)











![ANSWERED] All three of the boron-fluorine single bonds in BF3 are - Kunduz ANSWERED] All three of the boron-fluorine single bonds in BF3 are - Kunduz](https://media.kunduz.com/media/sug-question/raw/59983136-1659772204.1988795.jpeg)